Congenital Bile Acid Synthesis Defect
Primary bile acid synthesis disorders are a group of genetic metabolic disorders caused by various enzyme deficiencies involved in primary bile acids synthesis: cholic acid and chenodeoxycholic acid.1
They are rare autosomal recessive diseases that can present as neonatal cholestasis, advanced liver disease and/or fat and fat-soluble-vitamin malabsorption.1,2
The two most frequent primary bile acid synthesis disorders responsible for chronic liver disease are:
- 3ß-hydroxy-C27-steroid oxidoreductase deficiency (3β-HSD)
- Δ4-3 oxosteroid 5ß-reductase deficiency (Δ4-3-oxo-R)
They are characterised by an impaired production of primary bile acids (cholic acid and chenodeoxycholic acid) and the accumulation of atypical bile acid intermediates, which are hepatotoxic and cholestatic.
Unless treated, chronic liver disease usually progresses to cirrhosis and liver failure most of the time before adulthood.3
Bile acid synthesis disorders represent approximately 1% to 2% of all childhood cases of cholestasis.
3β-HSD and Δ4-3-oxo-R deficiencies have been reported in all regions of the world. According to a recent European survey, the minimum prevalence of these two conditions would be 1.13 per 10 million, the first being much more prevalent than the second (estimated prevalence of 0,99 per 10 million for 3β-HSD deficiency versus 0,14 per 10 million for Δ4-3-oxo-R deficiency).
The actual prevalence is probably higher, as these diseases are underdiagnosed.
(click on the image to see it in a better quality)
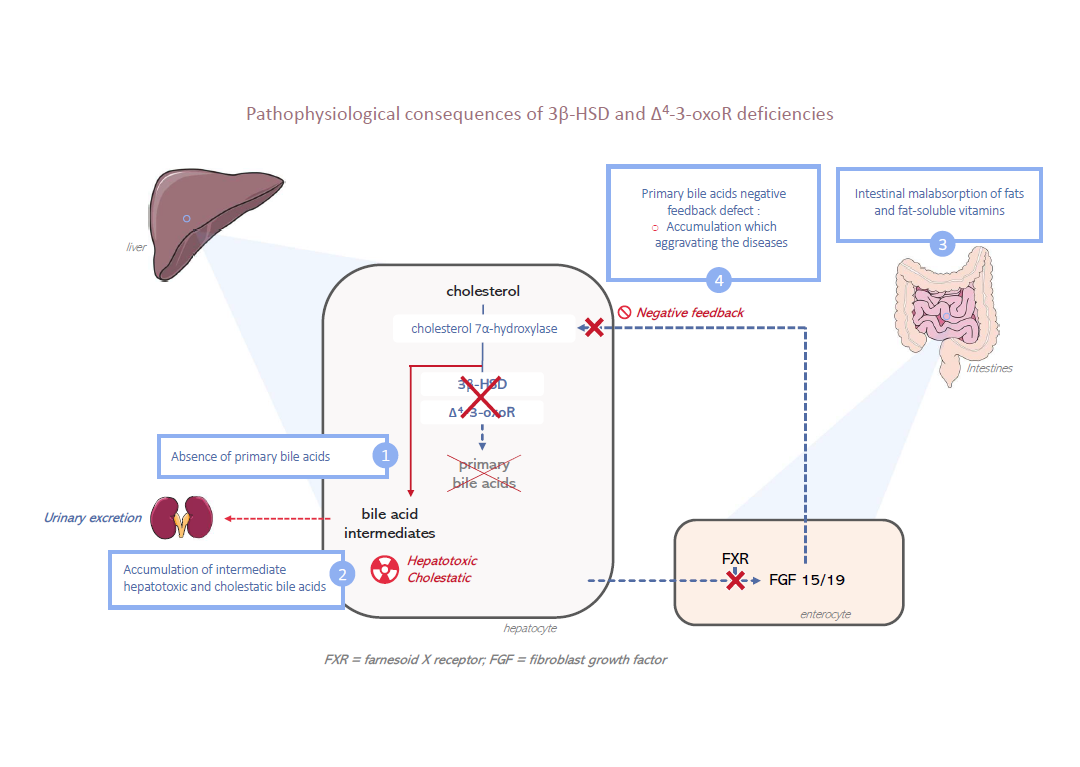
3β-HSD and Δ4–3-oxoR deficiencies result in an enzymatic block in the biosynthesis pathway of primary bile acids. Consequently, primary bile acids are not synthesized.
This absence directly and strongly decreases biliary secretion by reducing the bile acid–dependent fraction of bile secretion and results in a cholestasis with normal serum gamma-glutamyl transferase (GGT) activity.
The absence of primary bile acids in the intestine results in fat and fat-soluble vitamins (A, D, E and K) malabsorption.
Because of the enzymatic block, in hepatocytes there is an accumulation of bile acid intermediates that are hepatotoxic and cholestatic.
Finally, due to the lack of synthesis of primary bile acids, there is a suppression of the negative feedback loop that represses the biosynthesis pathway and further increases the production of these bile acid intermediates. These metabolites cannot be eliminated by the bile secretion, so they are eliminated in urine.
Their high concentration in the urine allows them to be identified and facilitates the diagnosis (see Diagnostic confirmation).
A possible diagnosis of 3β-HSD deficiency should be raised if all or part of the following clinical signs are present4:
- Cholestatic jaundice in new-borns or a more progressive onset of cholestasis in infants or small children;
- A mislabelled chronic (cholestatic) pathology of the liver with fibrosis developing towards cirrhosis;
- A lipid malabsorption syndrome (steatorrhea with no digestive or pancreatic pathology) causing fat-soluble vitamin deficiency, either symptomatic or not:
- Haemorrhagic manifestations of vitamin K deficiency
- Rickets or symptomatic hypocalcaemia of vitamin D deficiency
- Elimination of the osteotendinous reflexes and cerebellar syndrome related to vitamin E deficiency
- Ophthalmological manifestation of vitamin A deficiency (keratitis)
- Itching is absent;
- Hepatomegaly or hepatosplenomegaly.
This picture can occur in a context of consanguinity or history of non-identified chronic liver disease in siblings.
The routine blood lab test shows:
- Increased conjugated bilirubin;
- Increased transaminases;
- Normal gamma-glutamyl transferase (GGT) activity;
- And the most important sign is a normal total serum bile acids concentration in contrast with the clinical symptoms of cholestasis (discoloured stools etc.).
The clinical presentation of Δ4–3-oxoR deficiency is similar to 3β-HSD deficiency, but often earlier (first months of life). In contrast to 3β-HSD, infants with Δ4–3-oxoR deficiency tend to have more severe liver disease with rapid progression to cirrhosis and hepatic insufficiency.1
The diagnosis is confirmed based on biochemical and genetic arguments.
The diagnosis is initially confirmed based on a urinary bile acids analysis using gas chromatography combined with a mass spectrometry, or a comparable technique (FAB-MS, LC-MS). This test is done by specialised laboratories. It shows a specific urinary excretion profile for each bile acids synthesis disorder, providing a diagnosis.
In cases of 3β-HSD and Δ4–3-oxoR deficiencies, a metabolic urine test shows increased bile acids excretion, consistent with cholestasis, but qualitatively abnormal:
- Absence or low concentrations (traces) of primary bile acids;
- An abundance of atypical bile acid intermediates specific to each deficit (3β-OH-Δ5 in 3β-HSD deficiency and 3-oxo-Δ4 in Δ4–3-oxoR deficiency).
In cases of Δ4–3-oxoR deficiency, a metabolic urine test also shows the presence of allo-bile acid derivatives. Δ4–3-oxoR enzyme is affected by any advanced liver damage, regardless of its origin. A primary genetic Δ4-3-oxo-R deficiency is very likely if 3-oxo-Δ4 bile acids reach or exceed 70% of total urinary bile acids.
The diagnosis is then confirmed by a genetic analysis in molecular biology of the HSD3B7 gene encoding the 3β-HSD enzyme or the AKR1D1 gene encoding the Δ4-3-oxo-R enzyme.
Treatment of 3β-HSD and Δ4-3-oxo-R deficiencies are characterised by an excellent response to oral substitution treatment by a primary bile acid administered at a physiological dose. This treatment should be started straightaway so as to prevent an unfavourable development of the liver disease and avoid a liver transplant.
The lasting effects and good tolerability of this treatment promote compliance and allow patients to live a normal life. These facts are particularly important as the treatment must be administered for life: indeed, if the treatment with primary bile acid actually treats the hepatic disorder effectively, it is not correcting the underlying enzyme deficiency.
Cholic acid (major primary bile acid in humans) holds a marketing authorisation (MA) in France (and Europe) since 2013 for the treatment of 3β-HSD and Δ4-3-oxo-R deficiency and is currently the reference treatment for these diseases according to the majority of experts. In children and adults with these deficiencies the efficacy and safety of cholic acid is well known.1,4
Cholic acid restores the physiological feedback of the bile acids synthesis by activating the nuclear FXR receptor and inhibiting cholesterol 7α-hydroxylase, an enzyme limiting bile acids biosynthesis. It is also restoring the bile acid secretion by hepatocytes.
During the initial patient’s care, especially the diagnostic stage, a correction of any deficiencies in fat- soluble vitamins (A, D, E, K) by an oral or parenteral fat-soluble vitamin supplement may be needed.
Sources
This summary was prepared based on National Protocol for Diagnostic and Care: Primary bile acid synthesis disorders. October 2019. Coordinating Reference Centre for Biliary Atresia and Genetic Cholestasis in France.
- Heubi, J. E., Setchell, K. D. R. & Bove, K. E. Inborn Errors of Bile Acid Metabolism. Clin Liver Dis 22, 671–687 (2018).
- Sundaram, S. S., Bove, K. E., Lovell, M. A. & Sokol, R. J. Mechanisms of disease: Inborn errors of bile acid synthesis. Nat Clin Pract Gastroenterol Hepatol 5, 456–468 (2008).
- Jahnel, J., Zöhrer, E., Fischler, B., D’Antiga, L., & al. Attempt to Determine the Prevalence of Two Inborn Errors of Primary Bile Acid Synthesis: Results of a European Survey. J. Pediatr. Gastroenterol. Nutr. 64, 864–868 (2017).
- Gonzales, E., Matarazzo, L., Franchi-Abella, S., Dabadie, A., & al. Cholic acid for primary bile acid synthesis defects: a life-saving therapy allowing a favorable outcome in adulthood. Orphanet J Rare Dis 13, 190 (2018).
Find information about this rare disease by clicking on these links :